




 In vitro
Fertilization of Rat Eggs
In vitro
Fertilization of Rat Eggs
H. Miyamoto and M. C. Chang
Editor’s Note
Chinese American scientist Min Chueh Chang is probably best known for his work developing the oral contraceptive, but here he builds on a wealth of animal in vitro fertilization (IVF) research demonstrating IVF of rat eggs. Successful IVF of mammalian eggs had been demonstrated in many other species, and Chang himself had previously achieved IVF in rabbits by implanting black rabbit embryos conceived in the laboratory into a white rabbit. Critically, Chang acknowledged the importance of post-ejaculatory sperm maturation or “capacitation”, a phenomenon he had independently co-discovered with reproductive biologist Colin Russell Austin. Chang’s research paved the way for IVF of human eggs, culminating in the birth of the world’s first “test-tube baby”, Louise Brown, in 1978. 中文
AFTER the recognition of “capacitation of sperm” by Austin 1 and Chang 2 , successful in vitro fertilization of mammalian eggs was achieved in many species 3-17 . Although fertilization of rat eggs in vitro has been attempted 1,18-20 , incorporation of sperm into the vitellus was observed only after the dissolution of zona pellucida by chymotrypsin 19 . Here we report fertilization of intact rat eggs in vitro and the results obtained. 中文
Mature female CD strain rats were kept in a constant temperature room (23-25℃) with artificial light (19.00-7.00 h darkness) and their vaginal smears inspected daily. As ovulation usually occurs at about 2.00 h on the pro-oestrous to oestrous night 21 , the eggs were considered to be about 4 to 5 h and 7.5 to 8.5 h after ovulation when pro-oestrous rats were killed at 6.00 to 7.00 and 9.30 to 10.30 h respectively on the next day. Superovulation of mature rats was performed by i. p. injection of 30 IU of pregnant mare’s serum (PMS) on the morning of oestrus and 30 IU of human chorionic gonadotrophin (HCG), 52 to 58 h later; the rats were killed 14 to 16 h after injection of HCG. Oviducts were placed under light paraffin oil, equilibrated with 5% CO 2 in air in the presence of a small volume of saline, in a watch glass kept at 37℃ and the eggs in cumulus clot were released by dissecting the ampular portion of the oviducts. Sperm for insemination were prepared. The eggs were inseminated with sperm prepared as in Fig. 1. 中文
After incubation for 8 to 12 h, the eggs were removed, mounted on a slide and examined. They were then fixed with neutral formalin and stained for the assessment of fertilization. The eggs which had sperm within their perivitelline space (supplementary sperm) were defined as “penetrated” eggs. When these eggs had either enlarged sperm head(s) or male pronucleus(ei) with fertilizing sperm tail(s) in or on the vitellus, they were considered as undergoing fertilization. 中文
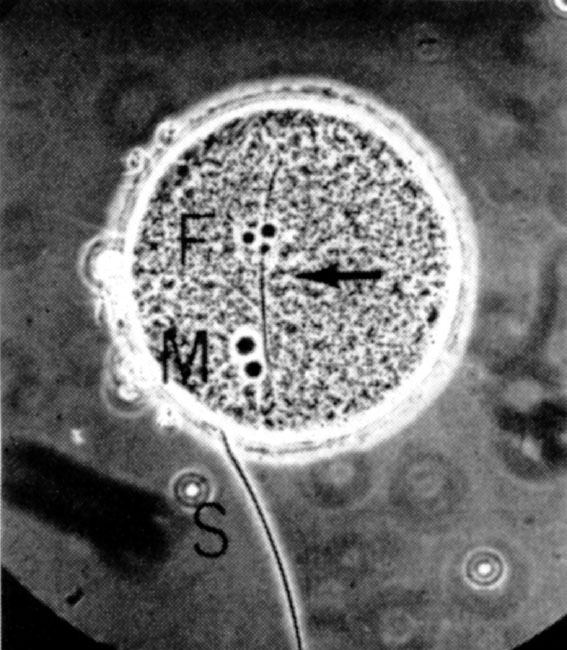
Fig. 1. A rat egg at pronuclear stage, fertilized in vitro , showing male pronucleus (M), female pronucleus (F), the fertilizing sperm tail (arrow) and a sperm attached to the zona pellucida (S). Photographed before fixation under a phase-contract microscope. Sperm for insemination were prepared either from the cauda epididymis or from the uterus of a female mated 0.5 to 1 h, or 10 to 11 h, previously. A drop of dense sperm mass was put into a watch glass and covered with 3 ml. of medium (modified Krebs-Ringer bicarbonate solution containing 114.2 mM NaCl, 4.78 mM KCI, 1.71 mM CaCl 2 ·2 H 2 O, 1.19 mM KH 2 PO 4 , 1.19 mM MgSO 4 ·7 H 2 O, 25.07 mM NaHCO 3 , 0.55 mM sodium pyruvate, 21.58 mM sodium lactate and 5.55 mM glucose, to which 4 mg ml. –1 of crystalline bovine serum albumin, 50 μg ml. –1 of streptomycin sulphate and 75 μg ml. –1 of penicillin G (potassium salt) were added). The p H value of the medium was adjusted to 7.4 to 7.5 by addition of 1 M NaOH and the final solution was filtered through a millipore filter to ensure asepsis. Blood of female rats was collected by heart puncture, allowed to clot and then centrifuged. Serum was sterilized by filtration through a millipore filter and stored under paraffin oil at 2 to 5℃ for no longer than five days. The uterine fluid was collected from oestrous rats by means of a syringe needle. Insemination was performed by adding 0.4 ml. of the sperm suspension to the egg clot under the oil, after removing oviducts and débris. In some cases the egg clot was washed three times with the same medium to remove the oviducal fluid. After adding 0.1 or 0.2 ml. of heated rat serum (at 56℃ for 40 min), the eggs and sperm were thoroughly mixed and incubated at 37℃ in an atmosphere of 5% CO 2 in air. The final concentration of sperm in the suspension was 1,000 to 4,000 sperm mm –3 .
Fig.1 shows a rat egg fertilized in vitro at the pronuclear stage. Table 1 shows that the eggs recovered from naturally-ovulated rats have a better chance ( P <0.01) of fertilization in vitro than those recovered from superovulated rats (highest penetration rates: 45% compared with 22%). The proportion of penetrated eggs was higher ( P <0.05) following insemination with sperm recovered from the uterus 10 to 11 h (17-45%) than 0.5 to 1 h after mating (7%) for both the naturally-ovulated and superovulated eggs. The eggs recovered 4 to 5 h after ovulation appeared to have a better chance of penetration (24-45%) than those recovered 7.5 to 8.5 h after ovulation (1-5%). By washing the egg clot before insemination, the proportion of penetration was decreased for both the naturally-ovulated (24% compared with 45%) and superovulated eggs (9-11 compared with 17-22%, not statistically significant), indicating that some beneficial factor may have been removed by washing. Although there was no obvious difference when different proportions of rat serum were added in the medium, sperm penetration was not observed when whole rat serum was used in a few experiments. Sperm penetration, however, was not observed in 52 naturally-ovulated eggs and 229 superovulated eggs when epididymal sperm, which had been pre-incubated for 2 to 7 h in the medium containing rat serum and uterine fluid, were used, they remained motile for longer than uterine-incubated sperm. This, and the higher proportion of eggs penetrated by sperm recovered from the uterus 10 to 11 h after mating demonstrated clearly that rat sperm do need capacitation in the female tract before they are capable of penetrating the egg. Hamster 6,8 , mouse 15 , guinea pig 17 and perhaps human 9,10 sperm can be capacitated in vitro , but we have shown the difficulty of capacitating rat sperm in vitro . Rat sperm is similar to rabbit sperm in this respect. 中文
Table 1. In vitro Fertilization of Rat Eggs
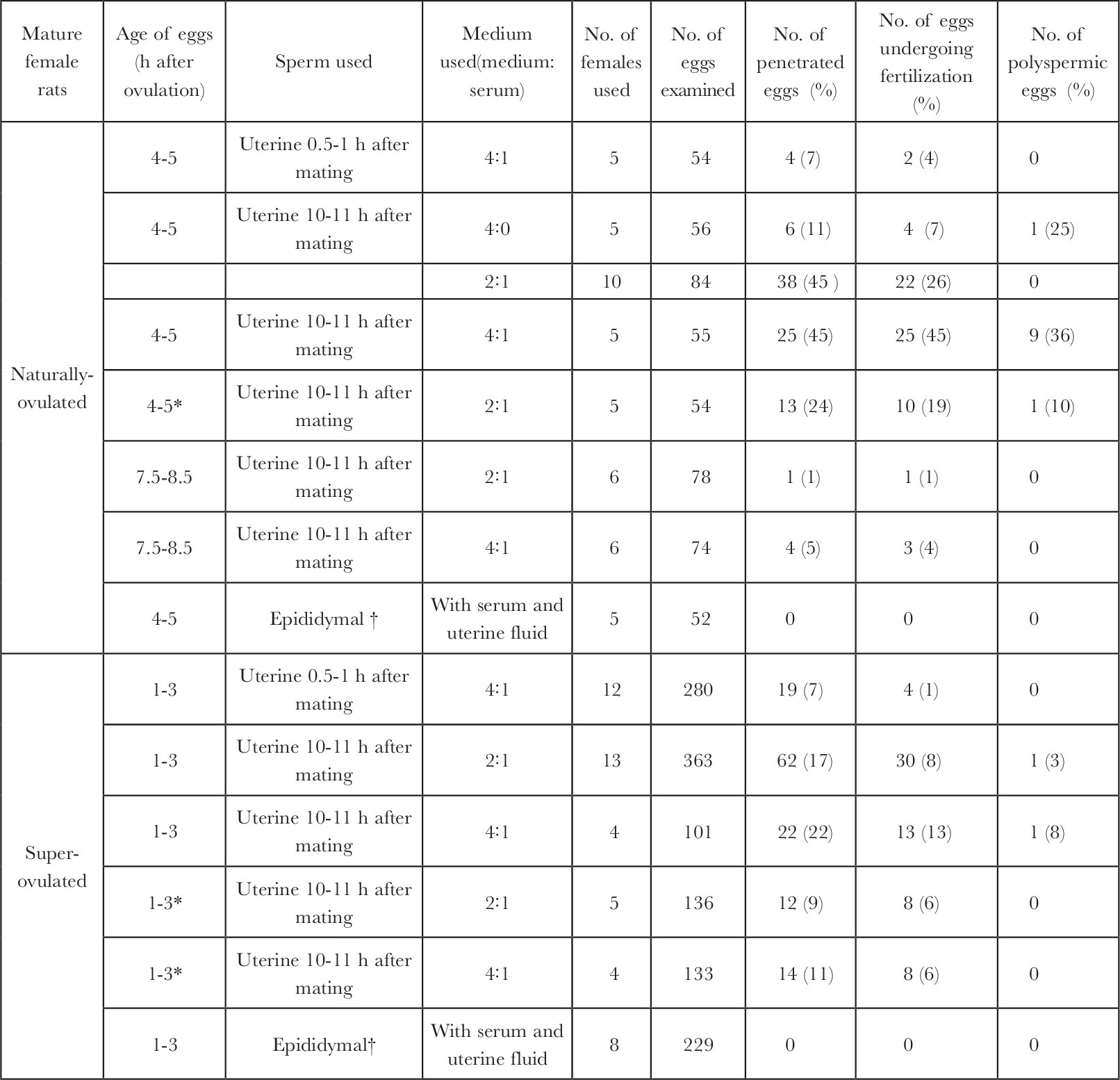
* Egg clot was washed three times before insemination.
† Epididymal sperm were incubated in the presence of uterine fluid for 2-7 h before insemination.
Of 126 eggs undergoing fertilization, 114 (90%) were monospermic and 12 (10%) were polyspermic; 12 had an enlarged sperm head and 114 had at least one male pronucleus. Fifty monospermic eggs and 12 polyspermic eggs had 1 to 26 supplementary sperm. Supplementary sperm (1 to 11) were also found in 88 unfertilized eggs, which indicates the occurrence of a vitelline block to sperm penetration as the eggs deteriorated in vitro . The incidence of polyspermy of rat eggs fertilized in vivo is about 1.2% 2 2 . The in vitro incidence of polyspermy is comparatively high as in the case of hamster 6 and mouse eggs 15 . Although fertilization of rat eggs in vitro was reported recently by Bregulla 20 , the method used, the photographs published, and the high frequency of cleavage of unfertilized rat eggs in vivo 23 , make his claim unconvincing. 中文
This work was supported by grants from the US Public Health Service and the Ford Foundation. We thank Mrs Rose Bartke for assistance. 中文
( 241 , 50-52; 1973)
H. Miyamoto and M. C. Chang
Worcester Foundation for Experimental Biology, 222 Maple Avenue, Shrewsbury, Massachusetts 01545
Received June 19; revised August 8, 1972.
References: