




阅读测试部分通常包含三或四篇文章。每篇文章后会有一组阅读理解题。以下为一篇例文。
1 Acids and bases are substances that form compounds and solutions with an electrical charge. When acids dissolve in water, they donate additional hydrogen ions to the solution. An acid, therefore, is a substance that increases the hydrogen ion concentration of a solution. A base, on the other hand, reduces the hydrogen ion concentration of a solution.
2 The strength of acids and bases is measured by using a numeric scale known as pH,a measurement that represents the number of hydrogen ions in a solution. pH is measured with a pH meter or with paper strips that have color indicators. The pH scale runs from 0 to 14, with the midpoint at 7. A neutral solution, such as pure water, has a pH of 7.0. A pH value of less than 7.0 denotes an acidic solution, and a value above 7.0 denotes a basic, or alkaline, solution. The pH of a solution declines as the concentration of hydrogen ions increases: the lower the number, the more acidic the solution. For example,a solution with a pH of 4.5 is far more acidic than one with a pH of 6.0. The pH for bases, or alkalis, is above 7.0: the higher the number, the greater the basicity or alkalinity.A pH of 8.5 is more alkaline than a pH of 7.5.
3 The internal pH of most living cells is close to 7.0. Most biological fluids measure within the pH range of 6.0 to 8.0. There are a few exceptions, however, including the strongly acidic digestive juice of the human stomach, which has a pH of about 2.0. The chemical processes of living cells are very sensitive to the concentrations of hydrogen ions. Biological fluids resist changes to their pH when acids or bases are introduced because of the presence of buffers, substances that minimize changes in the concentrations of these ions. Buffers in human blood, for example, normally maintain the blood pH very close to 7.4 because a person cannot survive very long if the blood pH drops to 7.0 or rises to 7.8.
4 Acids and bases are used in food preparation and in industrial processes. They can be very dangerous, causing burns and other injuries to people and animals, as well as damage to the environment, so they must be used properly and handled with care. Acids are very important substances. They cause lemons to taste sour, they digest food in the stomach, and they dissolve rock to make fertilizer. They also dissolve tooth enamel to form cavities. Vinegar is a weak acid, a dilute solution of acetic acid used in food preservation. Lemon juice (citric acid) is added to foods and beverages to give them a sour flavor. Other acids have agricultural uses, such as hydrochloric acid—also known as muriatic acid—which is used as a fertilizer for acid–loving plants. Bases, or alkalis,have a bitter taste and a slippery feel. Most hand soaps and commercial products for unclogging drains are highly basic. Household ammonia and lye are bases. Slaked lime(calcium hydroxide) is a base that is used in cements and paints.
5 Most plants and animals have preferred pH ranges, where they attain their best growth and health. Acid materials can be made less acidic by adding basic materials to them. In the pH management of soil, compounds that are basic—like slaked lime or crushed limestone—are added to the soil to raise its pH. Limestone has a pH of about 8.2, which will lower the acidity of acid soil.
对于这类多项选择题,考生需从四个选项中选出最佳答案。如下所示:
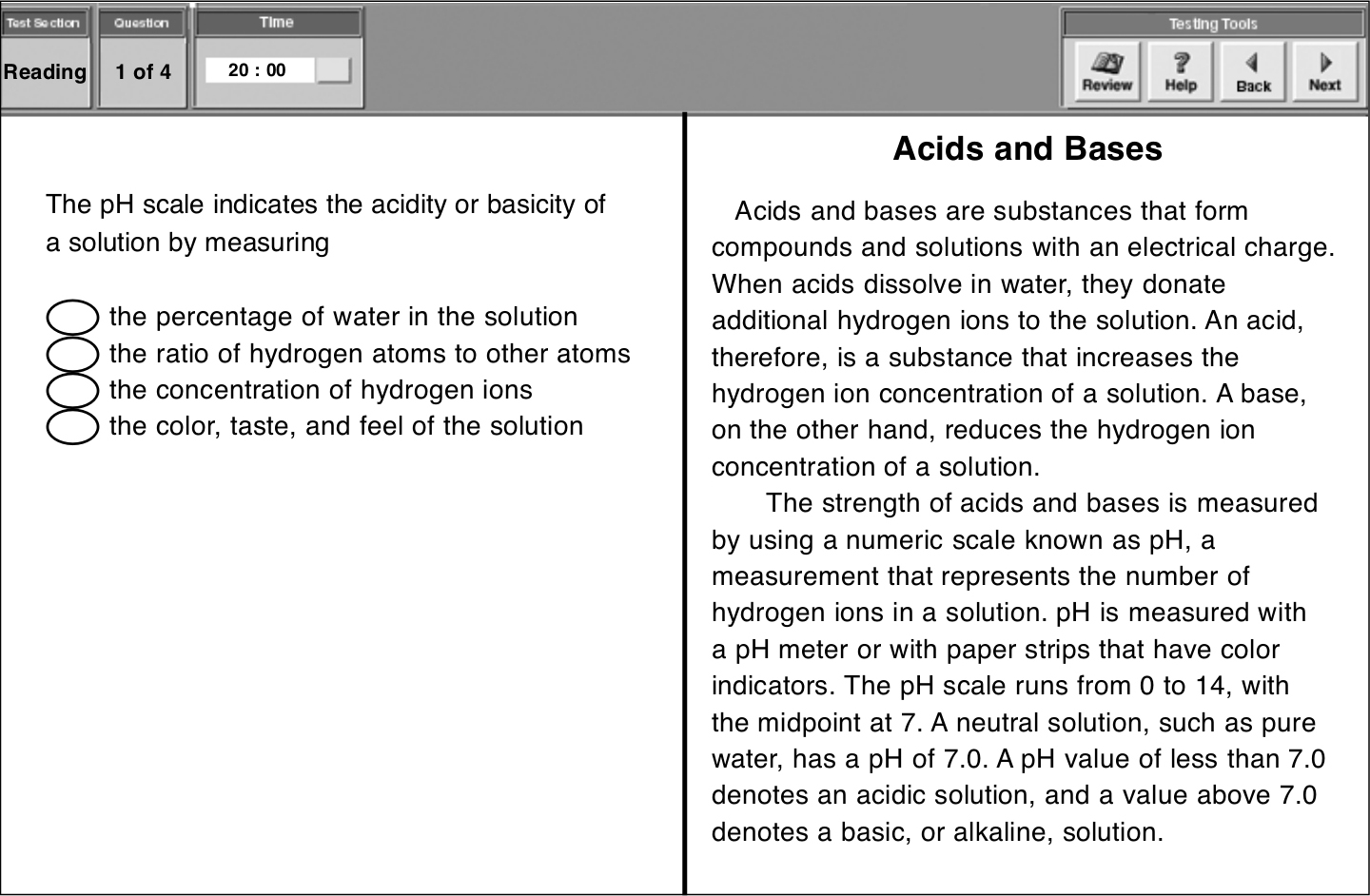
pH值通过测量溶液中氢离子的浓度(the concentration of hydrogen ions)来显示酸碱强度。因此,考生需点击第三个选项前的椭圆。
点击椭圆之后,该椭圆即变黑。要更改答案则点击其他椭圆即可。当考生确定所选答案无误时,可点击Next进入下一题。
对于这类题型,考生需点击方块将句子添入文章。如下所示:
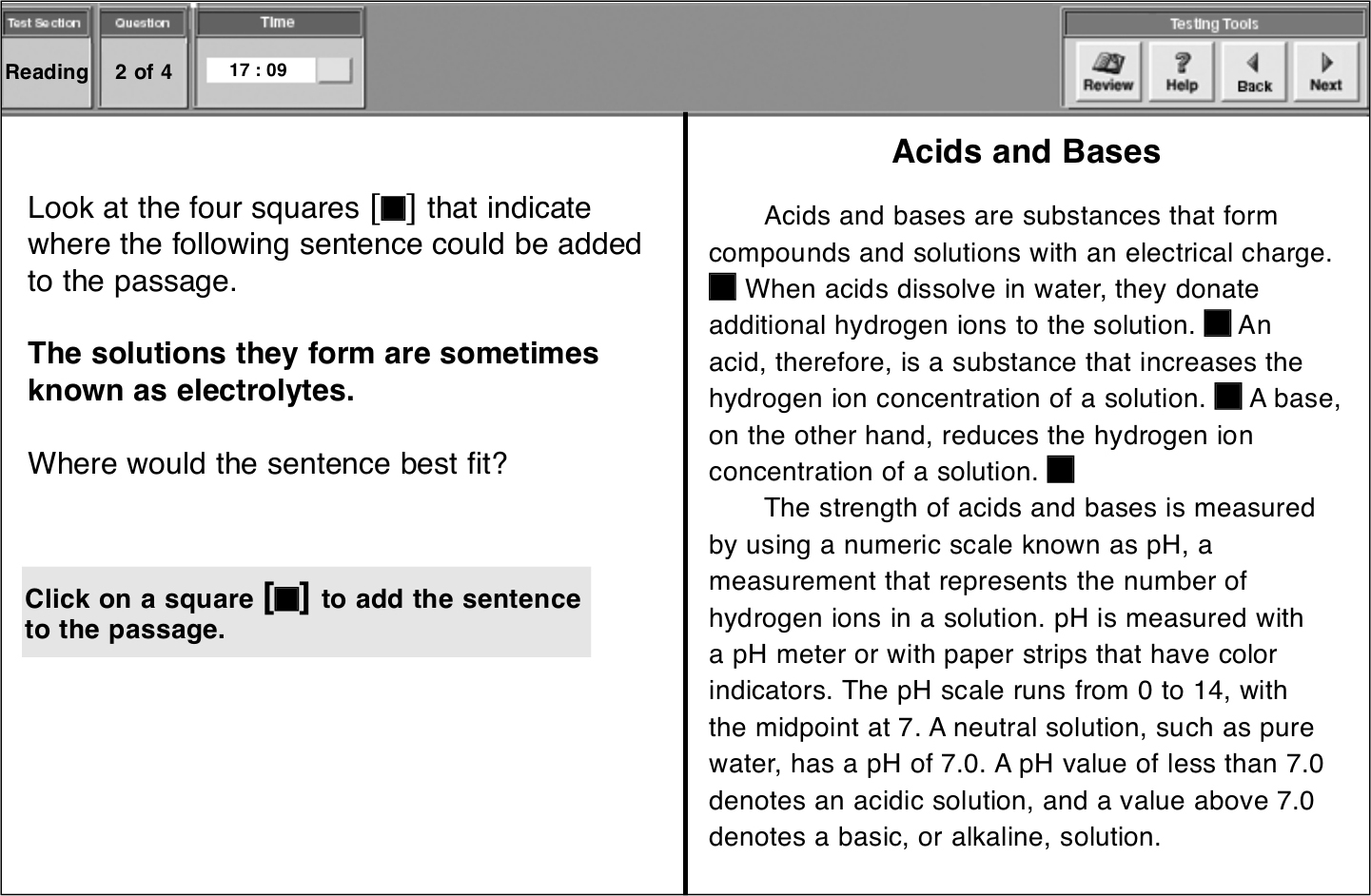
这个句子最适合第一个方块处。整段文字为:
Acids and bases are substances that form compounds and solutions with an electrical charge. The solutions they form are sometimes known as electrolytes. When acids dissolve in water, they donate additional hydrogen ions to the solution. An acid, therefore, is a substance that increases the hydrogen ion concentration of a solution. A base, on the other hand, reduces the hydrogen ion concentration of a solution.
点击方块后,句子即会出现在方块所在的位置。需修改答案时,点击其他方块,句子即出现在新的位置。当考生准备好进行下一题时,点击Next。
对于这类题型,考生需用鼠标拖动文字以完成摘要或表格。如下所示:
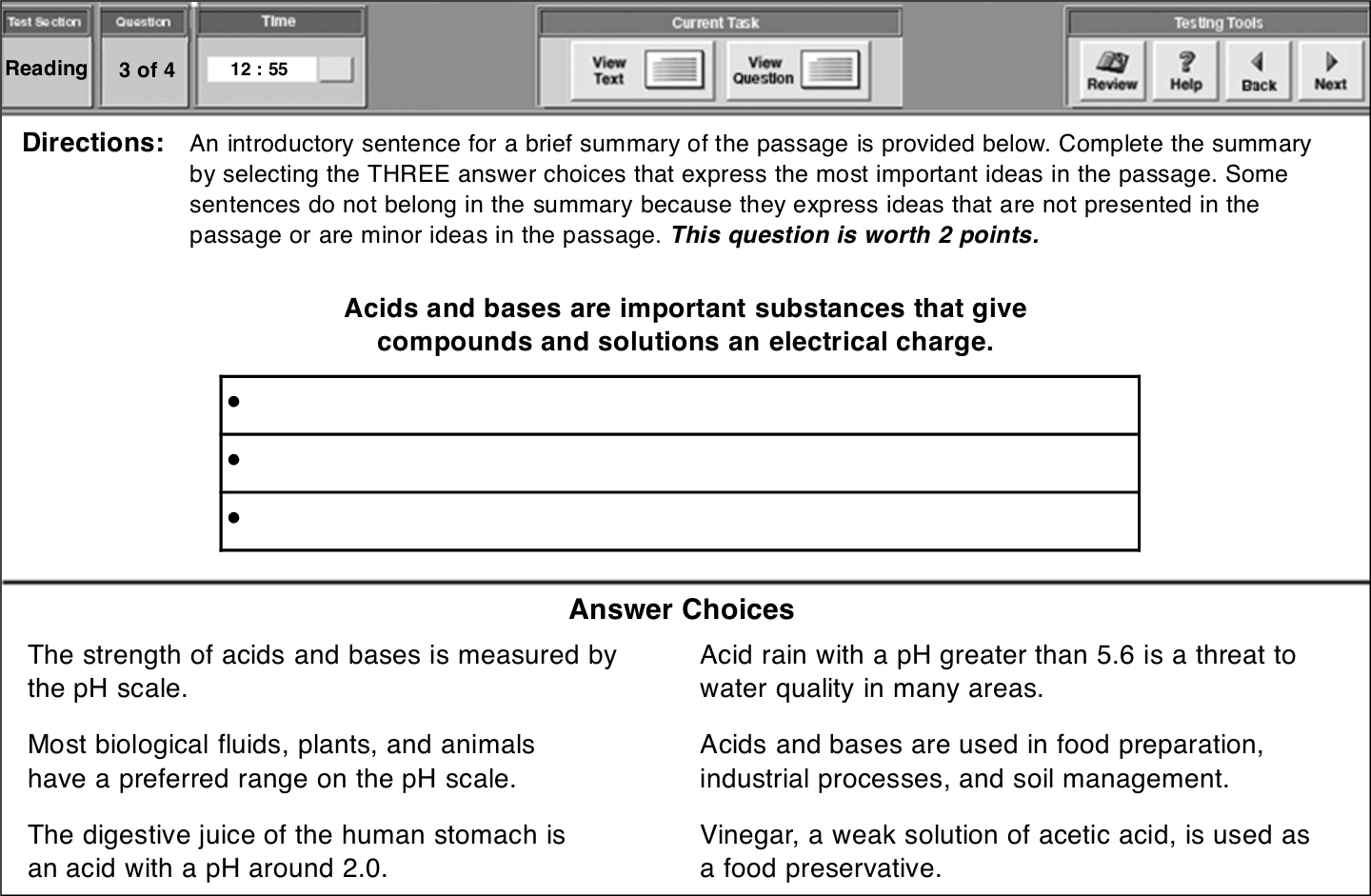
这篇文章最重要的三个观点为:
The strength of acids and bases is measured by the pH scale.
Most biological fluids, plants, and animals have a preferred range on the pH scale.
Acids and bases are used in food preparation, industrial processes, and soil management.
为了完成这篇摘要,把光标放在你想要移动的文字上,点击并按住鼠标把句子拖到正确的位置,然后句子就会出现在那里。要更改答案,对其点击即可,然后把新答案拖到正确的位置。
选对三个会得2分,选对两个得1分。只选对一个或都没选对不得分。
下面是另一个例子,同样需要用鼠标拖拽文字,如下所示:
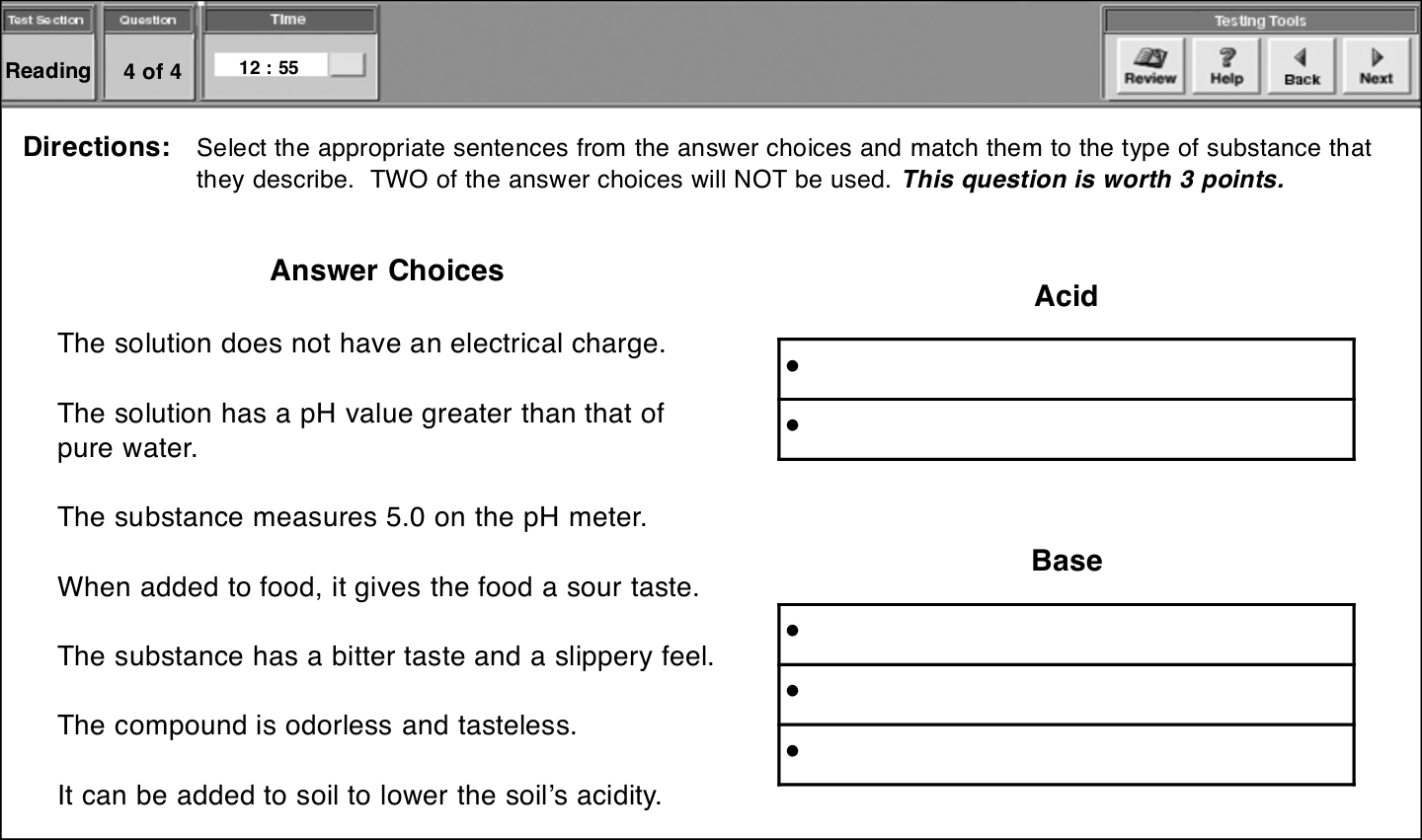
正确答案为:
Acid
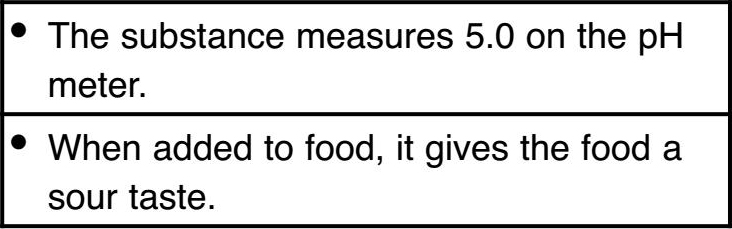
Base

答题时,把光标放在你想要移动的文字上,点击并按住鼠标把句子拖到正确的位置。本题分值为3分。选对五个会得3分,选对四个得2分,选对三个得1分。